Moderna Begins Human Study Of MRNA-Based Flu Shot After Covid Success
Share to FacebookShare to TwitterShare to LinkedinTopline Moderna announced Wednesday it has dosed the first participants in a study of what the vaccine manufacturer hopes will be a more effective flu shot based on the mRNA technology used to make its uber successful Covid-19 vaccine.
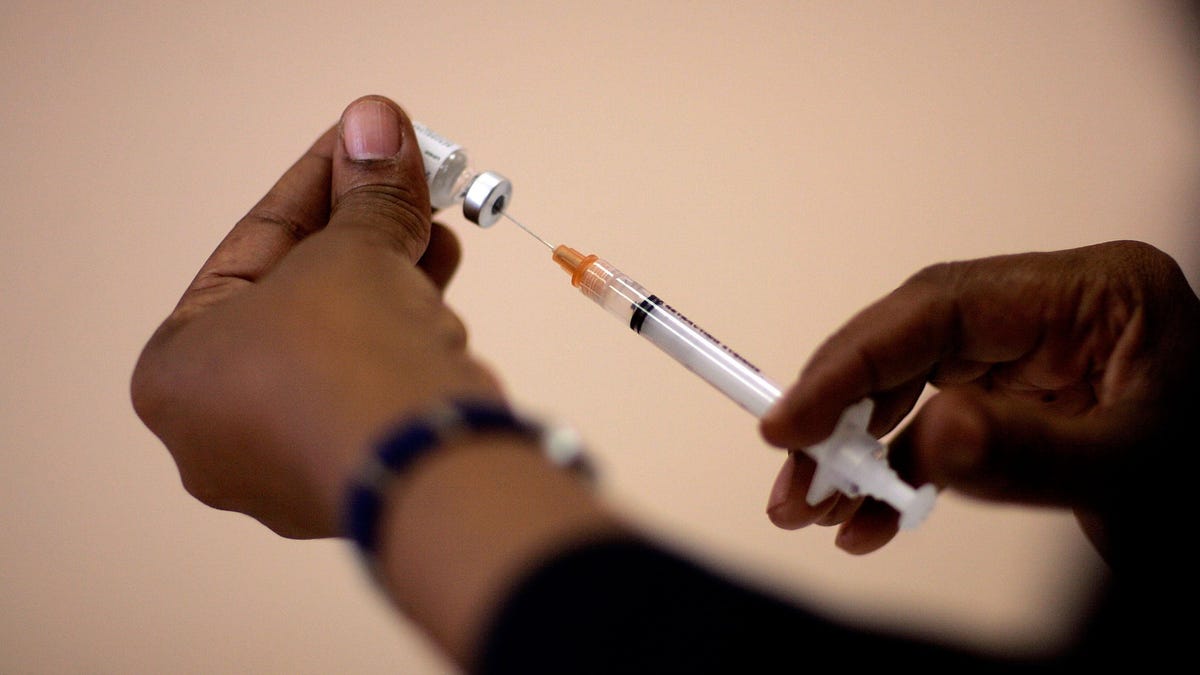
Barbara Dale, a school nurse, prepares an immunization needle for a child August 8, 2007 in Hialeah, … [+] Florida.
Getty Images Key Facts The biotechnology company said in a Wednesday morning press release that the Phase 1/2 clinical trial of its mRNA-based seasonal flu vaccine is underway.
This is the first time Moderna is testing this technology—which was used to develop both Moderna and BioNTech’s coronavirus vaccines in record time—for a flu vaccine on humans.
The mRNA flu vaccine candidate is designed to prevent multiple strains of influenza with much higher efficacy than current flu vaccines, according to the press release.
The company also said it has the eventual goal of creating an mRNA-based “combination vaccine” that would protect people against the flu, Covid-19 and other respiratory infections with one shot.
Crucial Quote “Our vision is to develop an mRNA combination vaccine so that people can get one shot each fall for high efficacy protection against the most problematic respiratory viruses,” CEO Stéphane Bancel said in a statement.
Key Background Flu vaccines take around six months to make and are only about 30-60% effective at preventing the seasonal illness. The World Health Organization (WHO) estimates anywhere from 290,000 to 650,000 die each year globally from flu-related respiratory deaths. In addition to rapidly expediting the time it would take to produce the flu vaccine, Bancel previously told Forbes he believes an mRNA alternative could be 90% effective or more.
Tangent Moderna isn’t the only company in the race to create a better flu vaccine. Sanofi and Translate Bio started their own trial of a seasonal mRNA flu vaccine candidate last month. Meanwhile, Pfizer-BioNTech has also shown interest in jumping into the mix.
Further Reading “What’s Next For Moderna Post-Covid-19: CEO Stéphane Bancel Details mRNA Pipeline” (Forbes)
Full coverage and live updates on the Coronavirus
Follow me on Twitter. Send me a secure tip.










 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  USDC
USDC  Solana
Solana  TRON
TRON  Dogecoin
Dogecoin  Lido Staked Ether
Lido Staked Ether