FDA Authorizes Pfizer Covid Vaccine For Kids Ages 5 To 11
Share to FacebookShare to TwitterShare to LinkedinTopline The Food and Drug Administration has granted emergency use authorization to Pfizer-BioNTech’s coronavirus vaccine for children between the ages of 5 and 11, the agency announced Friday, opening the door for millions of kids to start getting the jab next month.
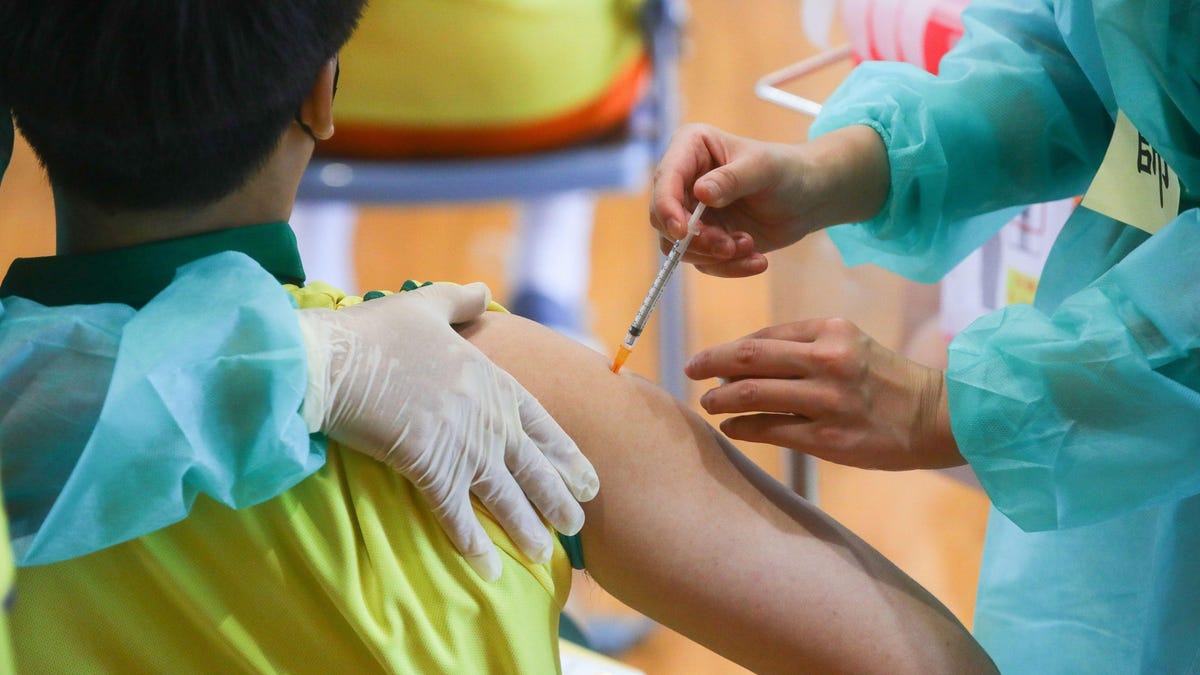
A child is injected with the Pfizer-BioNTech vaccine.
© 2021 Bloomberg Finance LP Key Facts The FDA authorized a two-shot course of Pfizer’s Covid-19 vaccine for children at about a third of the dosage used for teens and adults.
The agency’s authorization came days after an independent advisory panel deemed the vaccine safe and effective based on data presented by Pfizer, the FDA and the Centers for Disease Control and Prevention.
Pfizer’s clinical trial of the vaccine with this age group found the shot to be 91% effective at preventing infection and associated with “no significant adverse side effects.”
The vaccine is now awaiting final sign-off from the U.S. Centers for Disease Control, whose own panel of experts is scheduled to meet November 2 and 3.
Acting FDA Commissioner Janet Woodcock in a statement described the agency’s evaluation process as “comprehensive and rigorous,” emphasizing that parents and guardians should feel assured “this vaccine meets our high standards.
Crucial Quote Pfizer CEO and Chairman Albert Bourla praised the FDA’s decision as “another key marker in our ongoing effort to help protect families and communities, and to get this disease under control.”
Key Background The FDA’s advisory committee met Tuesday for a day-long meeting on authorizing the vaccine for children. The experts heard from company executives and health officials who offered evidence on the protections that could be offered by the vaccine, as well as its risks. Scientists from the CDC and FDA sought to quell concerns over a rare heart inflammatory syndrome known as myocarditis that has been linked to Covid vaccination. Dr. Matthew Oster, a pediatric cardiologist with the CDC, said his agency has not seen increased rates of the disease among vaccinated children between the ages of 12 and 17 and stressed that the disease is seen among those infected with Covid-19, too. Meanwhile, Dr. Leslie Bell of the FDA highlighted that there were no “significant adverse effects” among young kids who received Pfizer’s vaccine in early trials, and that the most common side effects were pain in the injection site, headaches and fatigue.
What To Watch For The White House said it has made plans to ensure the vaccine can be “quickly” and “conveniently” distributed as soon as it receives the final green light from the CDC. Administration officials have said they hope they can begin doling out the first doses as early as the first week of November.
Big Number 28 million. That’s how many children will be eligible for the Covid-19 vaccine once it’s approved for five- to 11-year-olds, the Biden administration said last week, noting that it has procured enough for everyone.
Further Reading “FDA Advisory Committee Recommends Authorizing Pfizer’s Covid Vaccine For Kids Ages 5 to 11” (Forbes)
“Pfizer Says Its Covid Vaccine Is 91% Effective At Preventing Infection For Young Kids” (Forbes)
“Here’s How The White House Plans To Distribute Children’s Covid Vaccines” (Forbes)
This is a breaking news story and will be updated.
Full coverage and live updates on the Coronavirus
Follow me on Twitter. Send me a secure tip.










 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  USDC
USDC  Solana
Solana  TRON
TRON  Dogecoin
Dogecoin  Lido Staked Ether
Lido Staked Ether