Pfizer Prepares To Seek U.S., EU Approval After Study Shows Vaccine Is Safe For Five To 11-Year-Olds
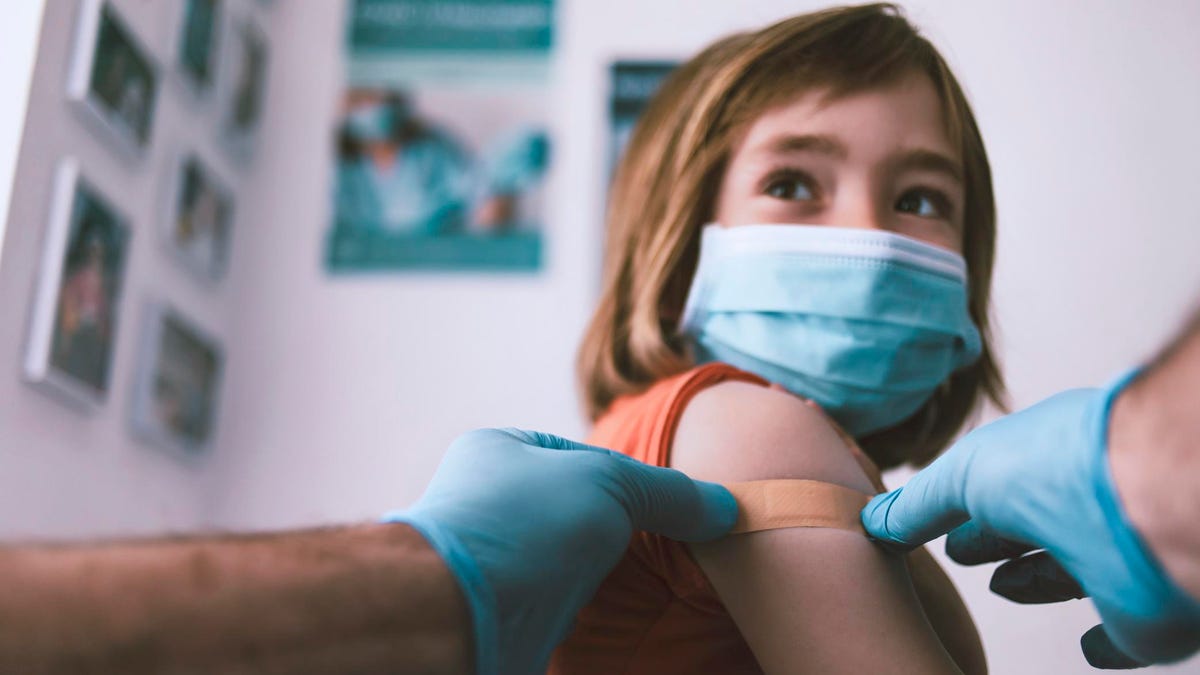
Share to FacebookShare to TwitterShare to LinkedinTopline Pfizer and BioNTech plan to ask regulators to give their Covid-19 vaccine emergency authorization for use in kids aged five and up, the companies said Monday, after announcing the first set of promising results from a major vaccine trial in this age group.
Pfizer said it would be asking to use its vaccine in children as young as five as soon as possible.
getty Key Facts Early findings from the trial, which involved 2,268 children aged five to 11 years old and has not been peer reviewed, showed the vaccine produced a “strong immune response” one month after the second dose, the companies said.
The children were given shots one third the strength (10 μg a dose) of what is used for those aged 12 and up, but the study indicated comparable immune responses to 16 to 25 year olds receiving the standard two-dose regimen, the companies said.
The vaccine is “safe” and “well tolerated” in the younger age group, the companies said, and side effects are also similar to those experienced by 16 to 25 year olds, including injection site pain, fever, fatigue and chills.
Pfizer CEO Albert Bourla said the results provide a “strong foundation” for the company to seek authorization for the vaccine’s use in children five to 11 years old, adding it plans to submit the findings to regulators like the U.S. Food and Drug Administration and the European Medicines Agency as soon as possible.
High-level findings from other parts of the trial, which cover children aged six months to five years, are expected by the end of the year, the companies said, and data from the full trial will be submitted for peer review and publication in a scientific journal.
Big Number 5.3 million. That’s how many children have tested positive for Covid-19 in the U.S. since the pandemic began, as of September 9, according to the American Academy of Pediatrics. The rate of pediatric infections has been on a steep incline since July and in the first week of September at least 250,000 children tested positive, making up a quarter of all new cases. Bourla said the rapid jump in pediatric cases and the threat the delta variant poses to children is “underscoring the public health need for vaccination.”
What To Watch For Moderna is also investigating the use of its Covid-19 vaccine in kids younger than 12.
Key Background While researchers show children and teenagers tend to have a much lower chance of developing severe illness from Covid-19, there are still clear benefits to vaccination. Children can and do develop life threatening illness from Covid-19 and pediatric hospitalizations have been rising across the country. These are more likely outcomes in the unvaccinated. Children are also at risk of developing long Covid, a debilitating condition that could last for months, though recent, inconclusive research suggests these risks may be smaller than previously believed. Illness aside, infected children are still capable of spreading the virus and keeping transmission going, including to adults who are at more risk of severe illness and death. Vaccines have been available to older teens in the U.S. for months, though none are yet authorized for use in those 12 and under. Global health agencies, like the World Health Organization, have slammed rich countries for expanding vaccination campaigns to children and other low-risk groups instead of sending vaccines to countries where even the most vulnerable have not been able to get a shot.
Further Reading Moderna Vaccine Highly Effective Against Delta—But Protection Against Infection May Wane Over Time, New Data Suggests (Forbes)
FDA Advisory Panel Recommends Against Pfizer Covid Boosters—Except For People Over 65 (Forbes)










 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  USDC
USDC  Solana
Solana  TRON
TRON  Dogecoin
Dogecoin  Lido Staked Ether
Lido Staked Ether